Asia Cervical Cancer Screening Market Size and Share Analysis – Growth Trends and Forecast Report 2024-2032
Published by Renub Research
Executive Summary
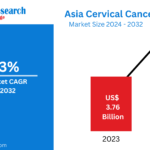
The Asia Cervical Cancer Screening Market is projected to reach US$ 5.46 Billion by 2032, up from US$ 3.76 Billion in 2023, growing at a CAGR of 4.23% during the forecast period 2024–2032. Rising public awareness, government initiatives, and advancements in screening technologies are reshaping cervical cancer prevention strategies across Asia. As countries push towards the WHO’s “90-70-90” elimination target, efforts around screening, vaccination, and early treatment are expected to intensify.
What is Cervical Cancer Screening?
Cervical cancer screening involves the detection of precancerous cells and early-stage cancer in the cervix to prevent disease progression. Key screening techniques include:
- Pap Smear Tests
- HPV DNA Testing
- Visual Inspection with Acetic Acid (VIA)
Routine screening enables early diagnosis, reduces cervical cancer incidence, and significantly lowers mortality rates.
Market Drivers
1. Government Initiatives and Policy Support
Governments across Asia are introducing national screening programs and HPV vaccination drives. India’s 2024-25 Interim Budget proposed HPV vaccination for girls aged 9-14. Countries like South Korea, Thailand, Singapore, and China have expanded their outreach via subsidized or free screenings, education programs, and public-private partnerships.
2. Technological Advancements
The adoption of liquid-based cytology, HPV DNA testing, and AI-enabled diagnostic tools has enhanced detection rates and efficiency. These innovations reduce the need for follow-up testing and improve access in rural areas through portable screening devices and telemedicine integration.
3. Rising Healthcare Expenditure and Infrastructure Growth
Countries like China, Japan, and India are investing heavily in healthcare. China’s health spending crossed ¥8.5 trillion in 2022. Rising income levels and private healthcare participation support the development of dedicated cancer care centers, driving the demand for cervical cancer screening services.
Market Restraints
- Limited Access in Rural Areas: Infrastructure and workforce limitations reduce reach in low-resource settings.
- Cultural Barriers and Stigma: Social discomfort and lack of education hinder participation in screening programs, especially in conservative rural communities.
- Cost Sensitivity: Advanced tests like HPV DNA testing are costlier than traditional Pap smears, limiting their adoption in public systems.
Country-Wise Market Analysis
China
China is aggressively expanding cervical cancer screening through its National Basic Public Health Service Project and Healthy China 2019–2030 Action Plan. Adoption of AI in cytology, rural outreach, and a growing HPV vaccination program support market expansion.
India
India is scaling up HPV testing and Pap smear screening through NGO initiatives and government programs. However, low awareness and stigma still limit coverage in rural areas. The government aims to vaccinate 90% of girls by age 15 and screen 70% of women by age 35 and 45.
Japan
Despite a robust national screening framework, screening participation remains low. Japan has adopted liquid-based cytology and HPV testing. Increasing focus is now placed on improving rural accessibility and AI-based molecular screening.
South Korea, Singapore, and Malaysia
These countries have well-organized screening programs with strong governmental and private healthcare sector collaboration. High awareness levels and innovation adoption place them ahead in Asia’s cervical cancer prevention landscape.
Indonesia and Thailand
These countries are advancing through VIA-based programs and scaling up HPV DNA testing. Public-private partnerships and global aid are vital in reaching underserved populations.
Related Report
- Oncology Precision Medicine Market Report By Indication (Lung Cancer, Stomach Cancer, Colorectal Cancer, Breast Cancer, Prostate Cancer, Liver Cancer, Esophagus Cancer, Cervical Cancer, Kidney Cancer, Bladder Cancer and Other Cancers), End Users (Hospitals, Diagnostic Centers, Research & Academic Institutes and Others), Region (North America, Europe, Asia, South America, Middle East and Africa), Company Analysis 2023-2028
- United States and Canada Cervical Cancer Screening Market, Forecast 2022-2027, Industry Trends, Impact of COVID-19, Company Analysis
- Cervical Cancer Screening Market, Size, Global Forecast 2023-2028, Industry Trends, Growth, Share, Outlook, Impact of Inflation, Opportunity Company Analysis
Market Segmentation
By Test Type
- Pap Smear Test
- HPV DNA Test
- VIA (Visual Inspection with Acetic Acid)
By Country
| Test Type | Countries Covered |
| Pap Smear | Japan, Korea, Singapore, Malaysia, India, China, Thailand, Indonesia |
| HPV DNA | Japan, Korea, Singapore, Malaysia, India, China, Indonesia |
| VIA | India, China, Thailand, Indonesia |
Key Company Profiles
| Company Name | Key Focus Areas |
| Abbott Laboratories | Advanced diagnostics, global outreach |
| Hologic Corporation | HPV testing platforms, AI integration |
| Becton, Dickinson | Cytology innovations, self-sampling kits |
| Siemens AG | Molecular diagnostics |
| Roche Diagnostics | HPV DNA tests, self-sampling devices |
| Quest Diagnostics | US-Asia diagnostics expansion |
| Cardinal Health | Preventive care diagnostics solutions |
Recent Developments:
- Feb 2024 – BD and Camtech Health launched Singapore’s first at-home cervical screening test.
- May 2024 – BGI Genomics launched a cervical cancer program in Rwanda.
- June 2023 – Asia-Pacific Women’s Cancer Coalition formed by Roche and partners to combat rising cancer rates.
- June 2022 – Roche launched HPV self-sampling kits for clinical use.
Future Outlook (2024–2032)
- Increased Screening Coverage: Focus on meeting WHO’s 90-70-90 goals across Asia.
- Expansion of HPV Vaccination: Accelerated by public funding and school-based immunization programs.
- Growth in Self-Sampling & At-Home Tests: Driven by patient convenience and privacy.
- Integration of AI: Enhances accuracy and cost-efficiency in diagnostics.
- Rural Outreach Programs: Through mobile health units and NGO collaborations.
Key Questions Answered in the Report
- What is the projected market size of the Asia Cervical Cancer Screening Market by 2032?
- What is the expected CAGR of the market from 2024 to 2032?
- What are the key drivers of growth in the Asia Cervical Cancer Screening Market?
- How are governments in Asia promoting cervical cancer screening?
- What role does technology play in improving cervical cancer screening?
- How does healthcare expenditure impact the cervical cancer screening market in Asia?
- What are the major challenges affecting the adoption of cervical cancer screening in Asia?
- Which countries are leading in cervical cancer screening programs in Asia?
- How does the HPV DNA test compare to the Pap smear in terms of effectiveness and cost?
- What role do NGOs and public-private partnerships play in expanding screening coverage?
Report Customization Services
- Region-Specific Analysis
- Segment Expansion (e.g., by Age Group or Test Setting)
- Strategy Consultation & Market Entry Plans
- Company Profiles (Up to 10 Additional Firms)
- Country-Specific Reports (On Request)
- Excel Dashboard & Editable Formats (PPT/Word)
Report Features
| Feature | Details |
| Base Year | 2023 |
| Historical Period | 2019–2023 |
| Forecast Period | 2024–2032 |
| Market Units | US$ Billion |
| Delivery Format | PDF + Excel (Editable PPT/Word on request) |
| Post-Sale Analyst Support | 1 Year |
| Customization Scope | 20% Free |
Pricing Options
- Single User PDF: $2,990
- Five User License + Excel: $2,990
- Corporate Multi-User License: $3,990
- Single User + Hard Copy: $3,490
- Dashboard (Excel): $2,490
Contact Us
📞 USA: +1-478-202-3244
📞 India: +91-120-421-9822
📩 Email: info@renub.com
🌐 Web: www.renub.com